The Pediatric Heart Network (PHN) is a group of hospitals across the United States, Canada and other countries that conduct research in children with congenital heart disease (CHD) or pediatric acquired heart disease and adults with congenital heart disease (ACHD).
The PHN was created and funded in 2001 by the National Heart, Lung and Blood Institute (NHLBI) to improve outcomes and quality of life in children with heart disease. More recently the mission has expanded to include research in adults with congenital heart disease. The PHN supports doctors and nurses to design and carry out clinical research so that these populations can receive high-quality, evidence-based care.
The PHN is made up of clinical research hospitals, a Data Coordinating Center, and NHLBI/NIH. Participating hospitals with experienced research teams that specialize in the care of patients with heart disease were chosen to become part of the PHN. Auxiliary sites are brought on for specific studies, depending on enrollment and expertise needed.
Since 2001, the PHN has conducted 29 studies, including 12 clinical trials, with sample sizes ranging from 20-1250 participants
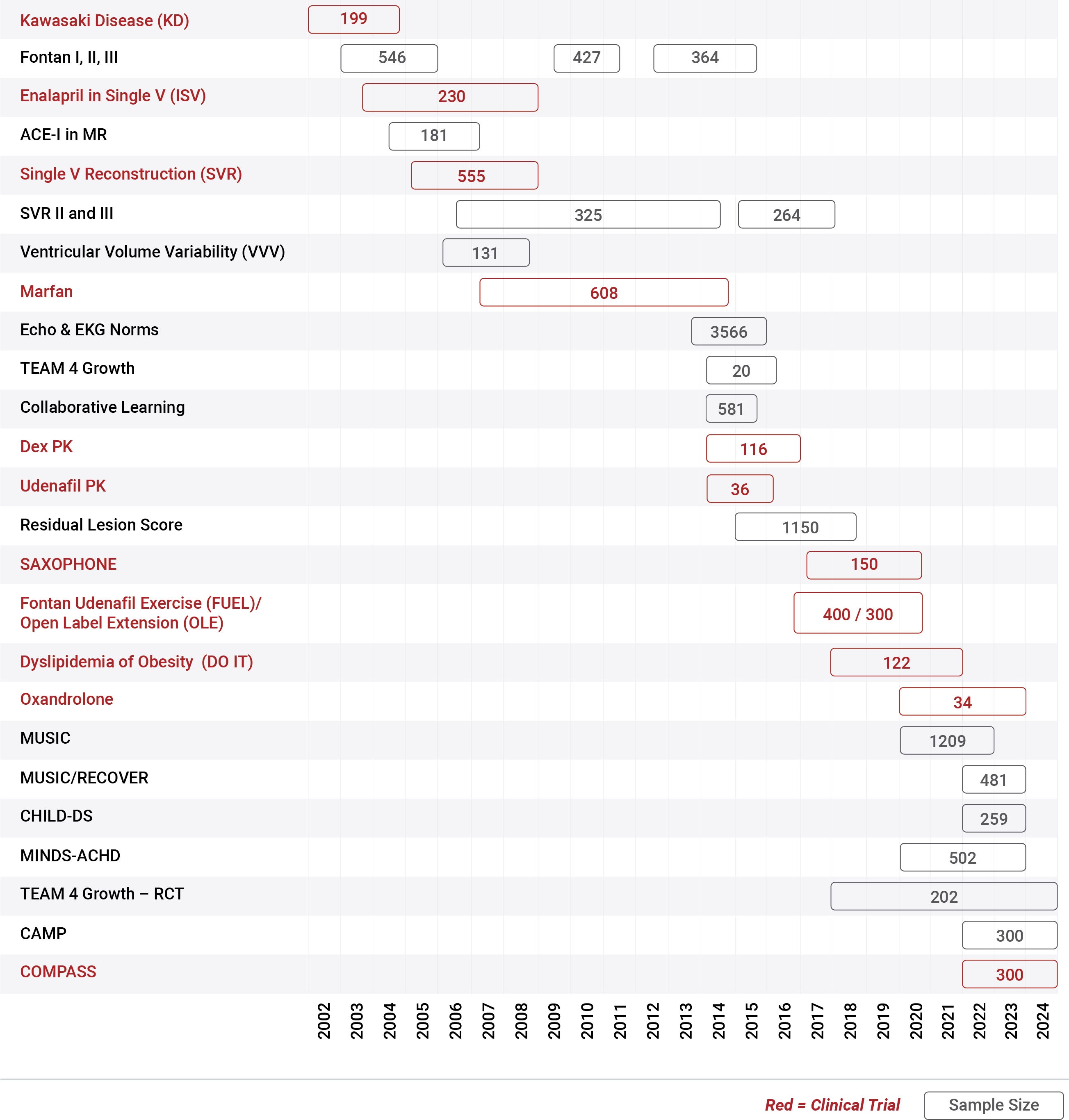
in individuals of all ages with congenital heart disease or pediatric acquired heart disease.
as the basis for improved evidence-based treatment options and standards of care.
new investigators.
during the conduct of excellent, ethical clinical research.
All PHN research centers do the same studies. This helps to increase the total number of patients available for each study. When studies need more patients, other skilled centers, called auxiliary sites, may be trained in one or more studies.
All of the centers carefully follow a study protocol and treat patients in similar ways so that the study results are accurate. Careful attention is given to How Studies are Created and Monitored when a study is being planned and done.
PHN nurses and doctors are skilled in the care of patients with heart disease and in the conduct of clinical studies. They have had special training in doing research in ways that help to protect patients in a study. They also have training in how to conduct the specific PHN studies and are sensitive to families with sick children. They can be a good resource for you as you decide whether to enroll yourself or your child in a research study.
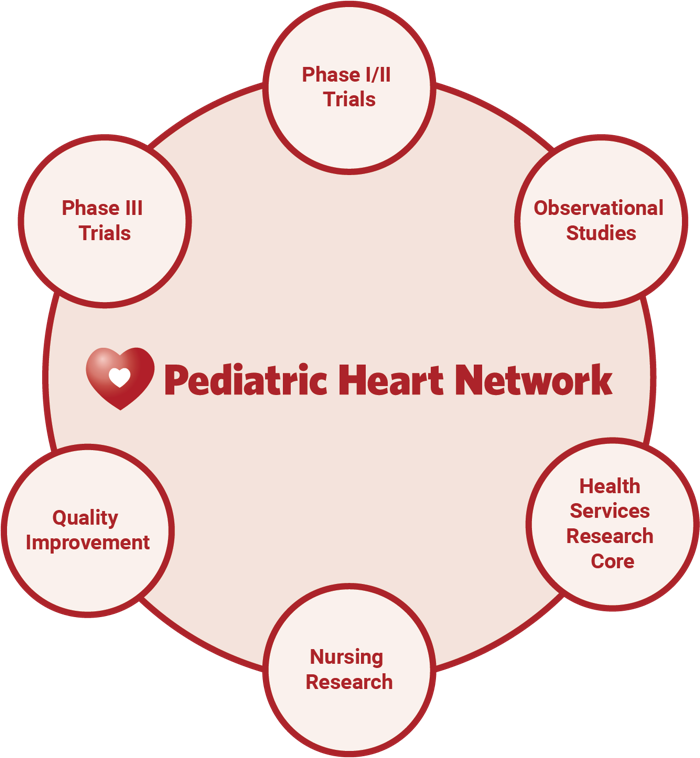
We currently have active studies in CHD, ACHD and dyslipidemia of obesity with participants ranging from birth to young adults. PHN conducts Phase I, II and III clinical trials, observational studies, quality improvement studies, nursing research and houses a health services research core.
These articles describe the structure and tasks of the PHN:
W. Lai, Am Heart J 2011; 161:13-67.
READ PUBLICATIONarrow_right_altJ. Kaltman, Circulation 2010; 121:2766-72.
READ PUBLICATIONarrow_right_altL. Mahony, Pediatr Cardiol 2006; 27:191-198.
READ PUBLICATIONarrow_right_altFor study results, view our complete publications list.
| Data Coordinating Center (DCC) |
|---|
| Julie Miller, MPH, PMP |
| Felicia Trachtenberg, PhD |
| Allison Crosby-Thompson, MSc |
| Melissa Joyce, CCRP |
| Lauren DiStefano, MBA |
| NHLBI |
|---|
| Gail Pearson, MD, ScD |
| Kristin Burns, MD |
| Bryanna Schwartz, MD, MPH |
| Vicki Pemberton, RNC, MS, CCRC |
| D'Andrea Egerson, RN |
| Lanre Ojese, MPH |
| Core Site | Principal Investigator |
|---|---|
| Boston Children’s Hospital | Sarah D. de Ferranti, MD, MPH |
| Children’s Hospital of Atlanta/Emory University | William Mahle, MD |
| Cincinnati Children’s Joint Heart Program Consortium (Cincinnati and U Kentucky) | James Cnota, MD |
| The GATHER Consortium (Colorado and Wash U) | Andrew Glatz, MD, MSCE |
| Medical University of South Carolina | Andrew Atz, MD |
| New York Consortium (Columbia and Mount Sinai) | Marc Richmond, MD, MS |
| University of Michigan | Caren Goldberg, MD |
| University of Pittsburgh | Bryan Goldstein, MD |
| University of Utah | Richard Williams, MD |